Looking for Module 5 - Equilibrium & Acid Reaction HSC Chemistry practice questions to test your knowledge before your exams? Here are 10 challenging questions for you to aid your studies.
Table Of Contents:
- Question 1
- Question 2
- Question 3
- Question 4
- Question 5
- Question 6
- Question 7
- Question 8
- Question 9
- Question 10
- Answer Key
- Conclusion
- Additional Helpful Resources
Question 1
Nitrogen dioxide (a brown gas) and dinitrogen tetroxide (a colourless gas) are both forms of oxides of nitrogen. They are in equilibrium according to the equation
2NO2(g) ⇌ N2O4(g).
An equilibrium mixture of the two gases at room temperature is light brown but at higher temperatures, the colour becomes a much deeper brown. What conclusion can be drawn from this observation?
A. The reverse reaction in the equation is endothermic.
B. The forward reaction in the equation is endothermic.
C. The brown colour is due to the strong nitrogen–oxygen bonds in NO2.
D. The equilibrium concentration of N2O4 is not dependent on temperature.
Question 2
What will happen when sulfuric acid is added to a saturated solution of sparingly soluble calcium sulfate?
A. The concentration of calcium and sulfate ions will increase over time due to the presence of H+ ions.
B. The concentration of calcium and sulfate ions will decrease over time due to the presence of H+ ions.
C. The concentration of calcium and sulfate ions will increase over time due to the presence of SO4 2– ions.
D. The concentration of calcium and sulfate ions will decrease over time due to the presence of SO4 2– ions.
Question 3
Calcium nitrate thermally decomposes to form calcium oxide, nitrogen dioxide and oxygen. The table below lists the thermochemical data for this reaction.
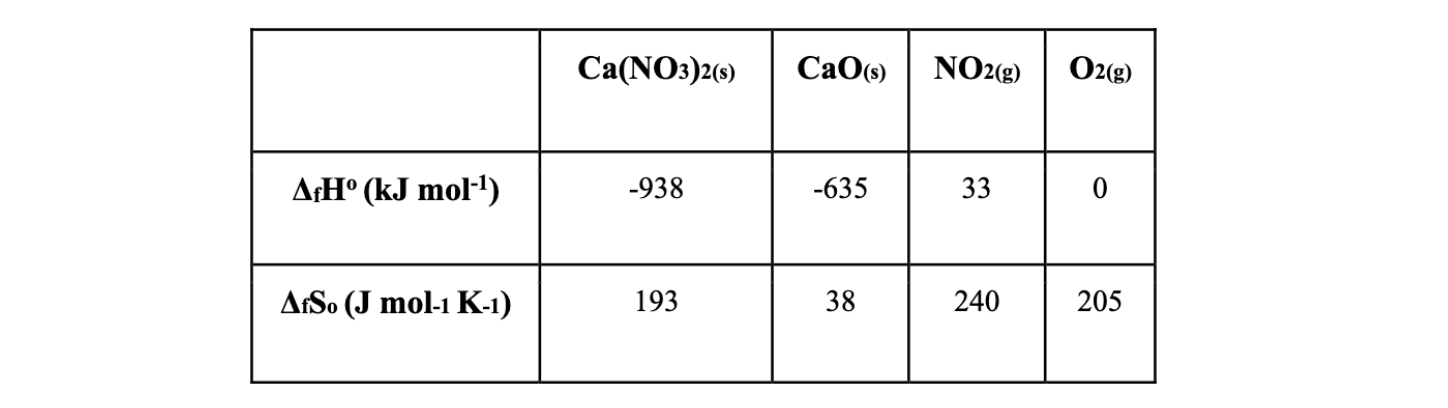
At what temperature will the reaction change from being non-spontaneous to spontaneous?
A. 863°C
B. 863 K
C. 8.63°C
D. 8.63 K
Question 4
The seeds of the cycad plant contain a toxin called cycasin. Indigenous Australians use techniques to detoxify these seeds to allow them to be eaten safely.
One method used in the detoxification process involved crushing the seeds to expose the inner kernels and then soaking the crushed seeds in water. What property of cycasin toxin does this method use?
A. The toxin is soluble in water
B. The toxin is insoluble in water
C. The density of the toxin is higher than water
D. The toxin can react with water
Question 5
An industrial plant makes ammonia from nitrogen gas and hydrogen gas. The reaction is exothermic. The graph shows the adjustments made to increase the yield of ammonia.
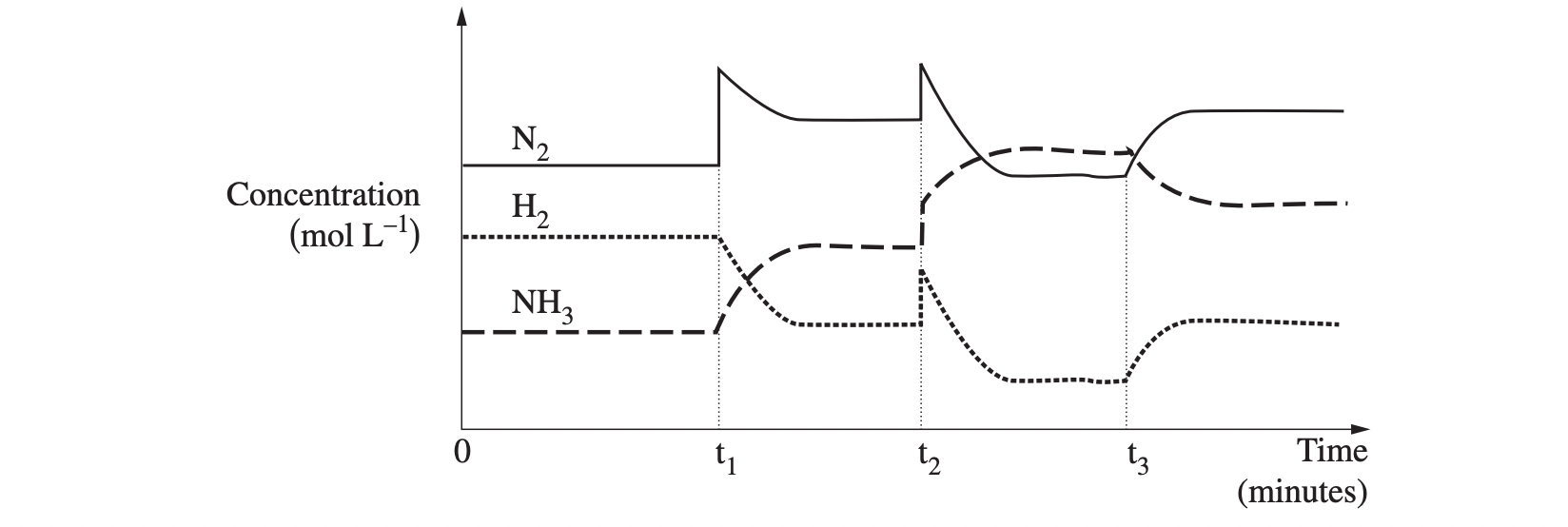
Account for the changes in conditions that have shaped the graph during the time the system was observed. Include a relevant chemical equation in your answer. (5 marks)
Question 6
When a sample of solid silver chloride is added to a 1.00 × 10−2 mol/L sodium chloride solution, only some of the silver chloride dissolves. Calculate the equilibrium concentration of silver ions in the resulting solution, given that the Ksp of silver chloride is 1.80 × 10−10. (3 marks)
Question 7
Determine whether silver sulfate will precipitate when 50 mL of 0.015 mol L-1silver nitrate and 20 mL of 0.010 mol L-1sodium sulfate are mixed at 25oC. The Ksp of silver sulfate is 1.20 x 10-5. (3 marks)
Question 8
Nitric oxide gas (NO) can be produced from the direct combination of nitrogen gas and oxygen gas in a reversible reaction.
(a) Write the balanced chemical equation for this reaction. (1 mark)
(b) The energy profile diagram for this reaction is shown.
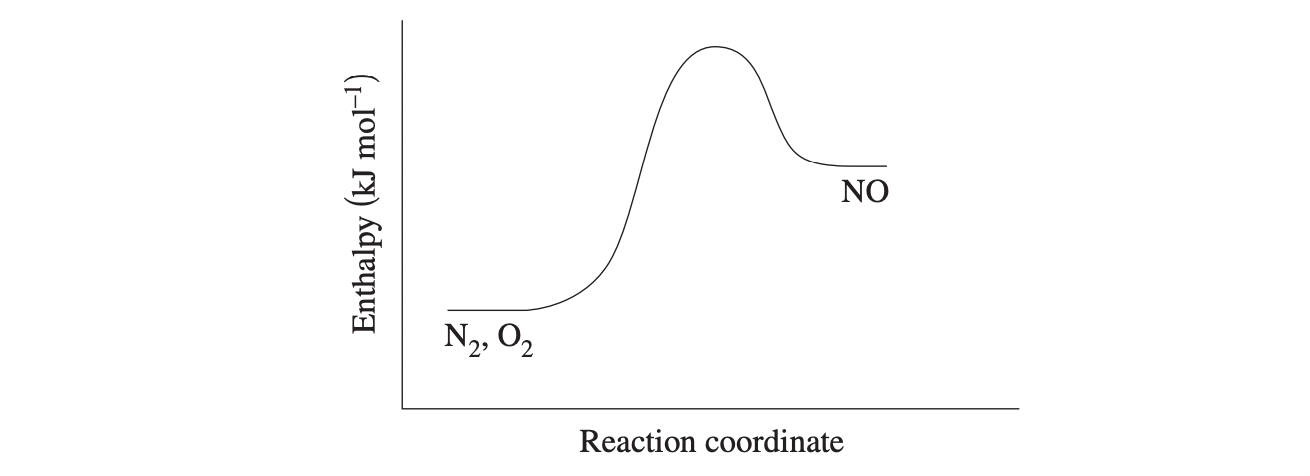
Explain, using collision theory, how an increase in temperature would affect the value of Keq for this system. Refer to the diagram in your answer. (4 marks)
Question 9
Ammonia is produced according to the following equilibrium equation.
N2(g) + 3H2(g) ⇌ 2NH3(g)
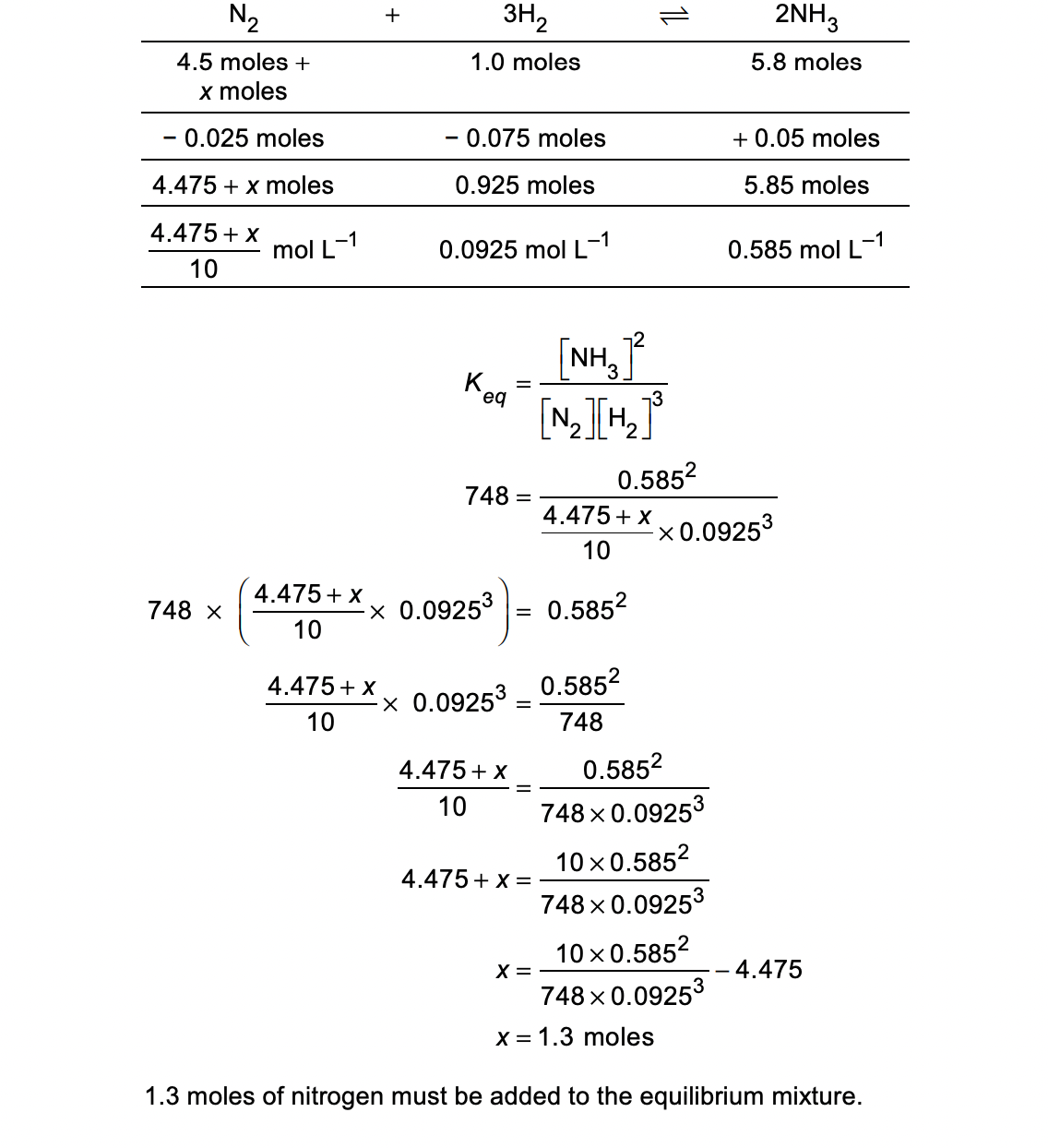
There are 4.50 moles of nitrogen gas, 1.00 mole of hydrogen gas and 5.80 moles of ammonia in a 10.0 L vessel. The system is at equilibrium at 298 K. The value of K eq at this temperature is 748. How many moles of nitrogen gas need to be added to the vessel to increase the amount of ammonia by 0.050 moles? (4 marks)
Question 10
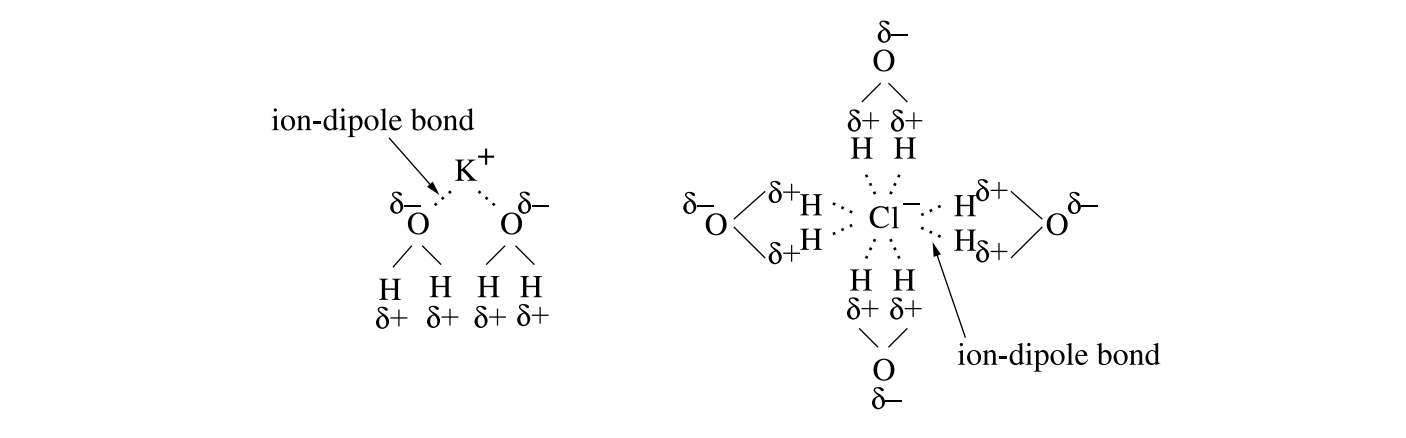
Describe the changes that occur in both bonding and entropy when potassium chloride is dissolved in water. Support your answer with a labelled diagram. (4 marks)
Answer Key
| Question | Answer |
| 1 | A |
| 2 | D |
| 3 | B |
| 4 | A |
Question 5:
N2(g) + 3H2(g) ⇌ 2NH3(g)
From t = 0 to t = t1, the system is at equilibrium. At t1 there is a sharp increase in the concentration of nitrogen which is likely to be due to more nitrogen being introduced to the system. According to Le Chatelier’s principle, when a system at equilibrium is disturbed, the equilibrium will shift in the direction that minimises the change. So the equilibrium will shift to the right to use up more nitrogen which will produce a greater yield of ammonia until equilibrium is re-established. At t2, the concentration of both reactants and products increases sharply, most likely due to a decrease in volume of the reaction vessel which will produce an increase in pressure on the system. In the above equation, it can be seen that there are 4 moles of gas on the left-hand side of the equation and 2 moles of gas on the right-hand side. An increase in pressure will cause the system to shift to counteract the change and thus move to the side with fewer moles of gas to reduce pressure. This increases the yield of ammonia as the forward reaction is favoured until equilibrium is re-established. At t3 there is a change to the system that favours the reverse reaction. As the concentration of reactants and products does not change suddenly, this is most likely to be due to a temperature change. As this reaction is exothermic, an increase in temperature is likely to have occurred which will favour the reaction that absorbs heat. Thus the reverse reaction is favoured, lowering the yield of ammonia until equilibrium is re‑established.
Question 6:
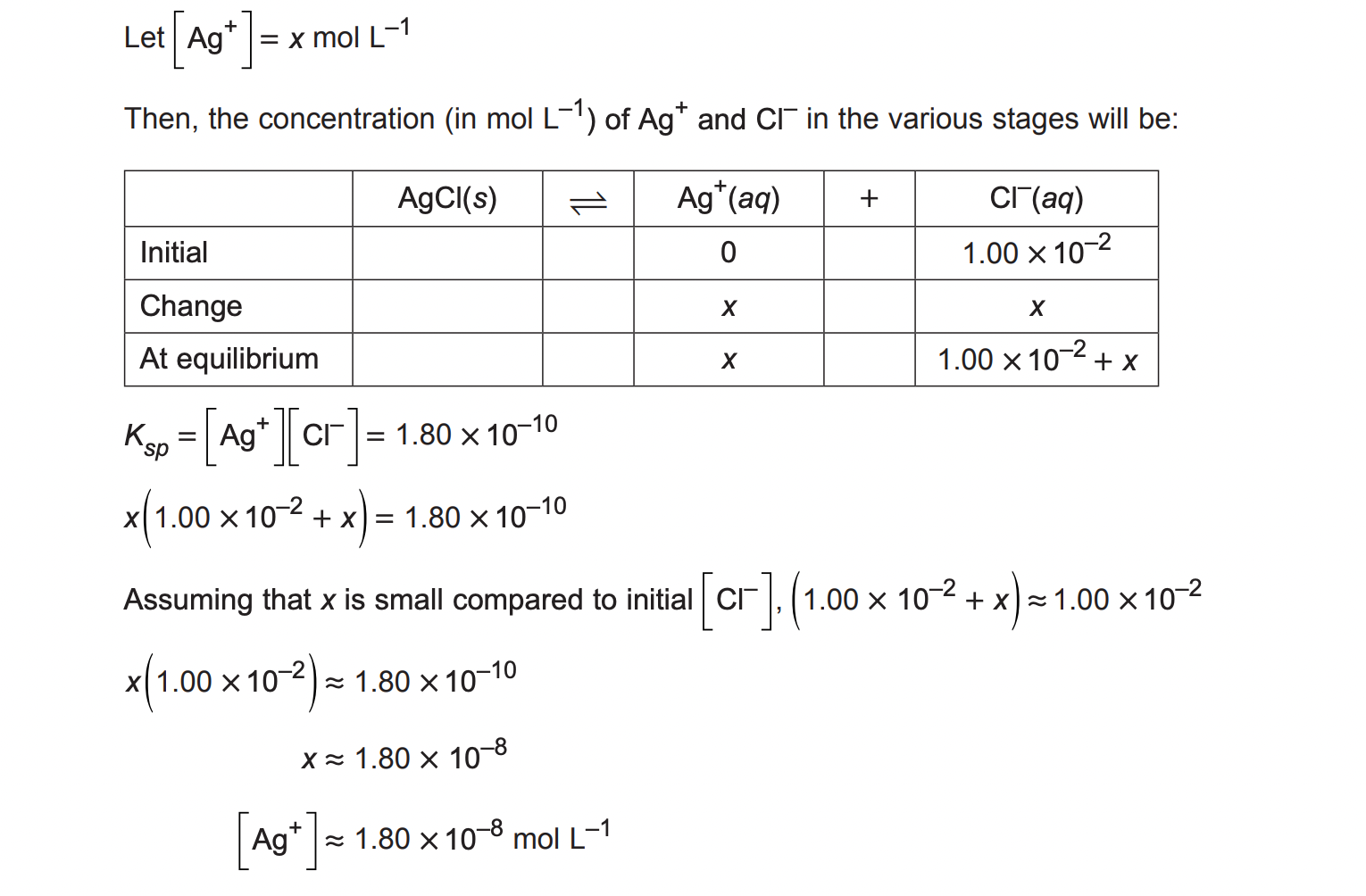
Question 7:
2AgNO3(aq) + Na2SO4(aq) → Ag2SO4(s) + 2NaNO3(aq)
AgSO4(s) ⇌ 2Ag+(aq) + SO2-,4(aq)
n(AgNO3) = 0.015/1000 x 50 = 7.5 x 10-4
[AgNO3] = 7.5 x 10-4 / 0.07 = 0.0107 M
n(Na2SO4) = 0.01/1000 x 20 = 2.0 x 10-4
[Na2SO4] = 2.0 x 10-4 / 0.07 = 0.00286M
Q = (0.0107)^2 x 0.00286 = 3.2798 < Ksp, ∴ no ppt forms.
Question 8: (a) N2(g) + O2(g) ⇌ 2NO(g)
Question 8: (b) The forward reaction is endothermic. For the reaction to proceed the reacting molecules must possess the activation energy to result in successful collisions. The activation energy for the forward reaction is greater than that of the reverse, exothermic reaction. An increase in temperature will increase the average kinetic energy of all molecules, resulting in more effective collisions increasing the reaction rate for both the forward and reverse reactions. However, the added temperature will have a greater impact on the forward reaction and the rate of this reaction will be higher than that of the reverse reaction. As a result, the forward reaction is favoured and K will increase.
Question 9:
Question 10:
Potassium chloride is an ionic compound soluble in water. Water is a polar molecule with hydrogen bonding between the molecules. The partially negatively charged oxygen in the water attracts the potassium ion and the partially positively charged hydrogen attracts the chloride ion, breaking the ionic bonds and forming ion‑dipole bonds. The entropy of the system is increased as the ordered lattice of the salt is broken and the ions disperse randomly throughout the solution.
What Our Students & Parents Say
600+ Five-Star reviews across all our tutoring programs — hear why below!👇
Conclusion
If you've made any mistakes with any of these questions, make a note of it, add it to your mistakes diary, and learn from that! All the best for your exams!
Additional Helpful Resources:



FAQs
1. What are Common Mistakes students make with this topic?
- Using initial concentrations instead of equilibrium values for Keq.
- Confusing the direction of equilibrium shift (not applying Le Chatelier correctly).
- Ignoring the number of gas moles in pressure/volume questions.
- Failing to show workings or explain reasoning in acid reactions.
2. What other Resources are available?
- Past year papers on NSW website.
- Tutoring from top HSC Chemistry Tutors.








